
It consists of a ceramic column with 99Mo adsorbed onto its top surface. The chemical separation device is called, in this example, a 99mTc Generator: So the nuclear facility produces the parent isotope which decays relatively slowly into the daughter isotope and the daughter is separated chemically from the parent at the hospital/clinic. The 99Mo is called the parent isotope and 99mTc is called the daughter isotope. Instead the nuclear facility supplies the isotope 99Mo which decays into 99mTc with a half life of about 2.75 days. This isotope has a half-life of six hours which is rather short if we wish to have it delivered directly from a nuclear facility. It involves obtaining a relatively long-lived radioisotope which decays into the short-lived isotope of interest.Ī good example is 99mTc which as we have noted before is the most widely used radioisotope in nuclear medicine today. This method is widely used to produce certain short-lived radioisotopes in a hospital or clinic. It consists of an ion gun for producing the charged particles, electrodes for accelerating them to high energies and a magnet for steering them towards the target material. The type of device commonly used for this method of radioisotope production is called a cyclotron. The target is exposed to the deuterons for a period of time and is subsequently processed chemically in order to separate out the 22Na nuclei.
#Nuclear fission generator plus
When it collides with a 24Mg nucleus a 22Na nucleus plus an alpha particle is produced. Some of these nuclei are of value to nuclear medicine.Īn example of this method is the production of 22Na where a target of 24Mg is bombarded with deuterons, that is: 24Mg + 2H → 22Na + 4He.Ī deuteron you will remember from chapter 1 is the second most common isotope of hydrogen, that is 2H. New nuclei can be formed when these particles collide with nuclei in the target material. Examples of such charged particles are protons, alpha particles and deuterons. In this method of radioisotope production charged particles are accelerated up to very high energies and caused to collide into a target material. One such reactor exists in Australia at Lucas Heights in New South Wales and many others exist throughout the world. The fission process is controlled inside a device called a nuclear reactor. Some of these new nuclei are of value to nuclear medicine and can be separated from other fission fragments using chemical processes. Following absorption of a neutron such nuclei break into smaller fragments with atomic numbers between about 30 and 65. This disintegration process can be induced to occur when certain heavy nuclei absorb neutrons.
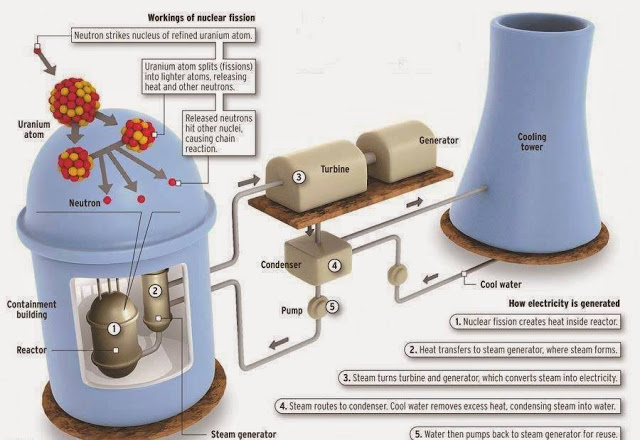
We were introduced to spontaneous fission in chapter 2 where we saw that a heavy nucleus can break into a number of fragments. The production methods we will consider are nuclear fission, nuclear bombardment and the radioisotope generator. Finally since the radioisotope needs to be incorporated into some form of radiopharmaceutical it should also be capable of being produced in a form which is amenable to chemical, pharmaceutical and sterile processing. For this reason most of the radioisotopes used emit gamma-rays of medium energy, that is between about 100 and 200 keV. From an energy point of view the gamma-ray energy should not be so low that the radiation gets completely absorbed before emerging from the patient's body and not too high that it is difficult to detect.

For this reason they generally have a short half life and emit only gamma-rays - that is no alpha-particle or beta-particle emissions.
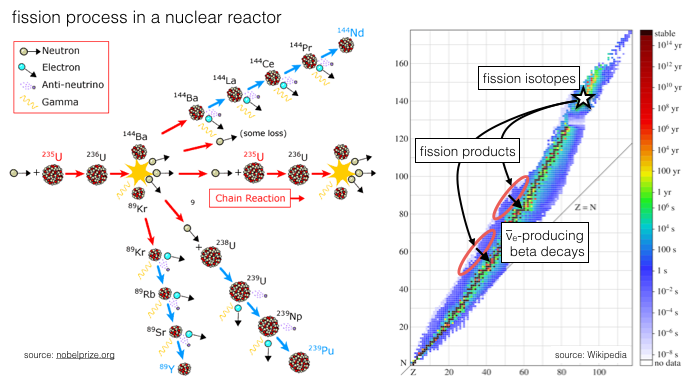
The type of radioisotope of value to nuclear medicine imaging should have characteristics which keep the radiation dose to the patient as low as possible. We return to sources of radioactivity in this chapter in order to learn about methods which are used to make radioisotopes. We have looked at the subject of radioactivity in earlier chapters of this wikibook and have then progressed to cover the interaction of radiation with matter, radiation detectors and imaging systems. As a result medical applications generally require the use of radioisotopes which are produced artificially. They also belong to elements which are not handled well by the human body. Most of the radioisotopes found in nature have relatively long half lives.


 0 kommentar(er)
0 kommentar(er)
